Ethics & Governance
All human research that takes place in NSW public health organisations must be ethically and scientifically reviewed and approved by a Human Research Ethics Committee in accordance with the National Health Medical Research Council National Statement on Ethical Conduct in Human Research (2007), and the Policy Directive Research – Ethical & Scientific Review of Human Research in NSW Public Health Organisations.
This includes but is not limited to clinical research, clinical trials, epidemiological research, health services research, population health research, and qualitative research.
In Australia, Coordinating Principal Investigators (CPIs) or other applicants must use the Human Research Ethics Application (HREA) form to apply for Human Research Ethics Committee consideration.
NSW supports single ethical review for studies taking place in more than one NSW public health organisation. NSW is also part of the National Mutual Acceptance scheme, where research taking place in public health organisations in more than one participating Australian jurisdiction needs only one ethical and scientific review. Some exemptions apply and are available on the National Mutual Acceptance page.
Human Research Ethics Applications to a NSW or ACT Human Research Ethics Committee should be completed in the Research Ethics and Governance Information System (REGIS) or in Online Forms. As sites are at different stages in their adoption of REGIS, check with your local research office for guidance before submitting your ethics and site assessment applications.
Human Research Ethics Committees
The role of Human Research Ethics Committees is to protect the welfare and rights of research participants. There are 22 HRECs that serve the public health system in NSW. A directory of HRECs can be accessed on the ethics and governance contacts page.
The NSW Ministry of Health supports and develops the network of NSW HREC through:
- information sharing
- education
- policy development
- mechanisms for the streamlining of processes
- development and implementation of policies to manage emerging research ethics issues.
Selecting a NSW Human Research Ethics Committee
For single centre research projects in NSW, an application should be submitted to the local HREC.
For multi-centre research projects where all sites are within NSW, an application may be submitted to any NSW Health lead Human Research Ethics Committee, provided that the Committee is accredited in the research area of the project. This applies to both full and expedited HREC review. For example, clinical trials must be submitted to a lead Committee accredited in clinical trials/interventional clinical research. In addition, if the lead Committee operates within a specialised therapeutic area, only research in that therapeutic area may be lodged with that Committee. Where possible, the application should be submitted to a lead Human Research Ethics Committee associated with one or more of the sites at which the research is to be conducted.
Human Research Ethics Application
The Human Research Ethics Application (HREA) is the National Health and Medical Research Council’s online ethics application form developed for all research involving humans. The HREA is designed to guide applicants through the ethical principles of the National Statement on Ethical Conduct in Human Research (2007).
The HREA aims to streamline and simplify the ethics review application process by only asking the questions that apply to the research in question. The HREA is applicable to all human research, from low-risk projects to complex multi-centre clinical trials.
All new ethics applications to NSW Human Research Ethics Committees, regardless of study risk level, must be completed on a Human Research Ethics Application (HREA) on the Research Ethics and Governance Information System (REGIS).
IMPORTANT: Please do not create a HREA for a NSW public health HREC at the NHMRC’s HREA website as the NSW Health ethics committee will not be able to import and review the application.
Resource: NHMRC Project Description-Protocol Template Source is HREA, or should be. This would be a useful resource for Research Office’s when discussing projects with Research communities.
NSW Early Phase Clinical Trials Framework
NSW Health has developed a framework to support early phase clinical trials across NSW. Following broad support from the sector and endorsement by local health district and specialty network Chief Executives, the Framework has been approved by the Chief Health Officer.
More than 100 experts across the sector were involved in consultations to inform the design of the Framework.
The Framework contains the design of two practical schemes to make NSW a centre of excellence in clinical trials, through the provision of a high quality and efficient environment to conduct early phase trials, with the ultimate aim of improving health outcomes for NSW residents.
More information on the Framework is available on the NSW Early Phase Clinical Trials Framework page.
Clinical trials roles and responsibilities
The Australia-wide Clinical Trials Jurisdictional Working Group has developed five documents to help clarify roles and responsibilities for organisations, institutions, investigators and sponsors who are engaged in clinical trials. While these documents focus on responsibilities relevant to clinical trials and should be reviewed within that context, there are some that may have wider application.
The documents are designed to help organisations develop appropriate competencies, position descriptions and training/induction programmes, develop ethics and governance policies, clarify investigators’ responsibilities, and facilitate the allocation and delegation of responsibilities for both externally (commercial/non-commercial) and internally (eg. investigator-initiated) sponsored trials.
The documents inform the Jurisdictional Working Group’s Key Priority Area of “Enhancing National Consistency for Ethics and Governance”. This aims to streamline and standardise clinical trial start-up processes to maximise efficiency and predictability. They will be used locally by NSW Health’s Office for Health and Medical Research, and nationally by the National Mutual Acceptance Scheme in order to guide policy in this area.
The guidelines outlining roles and responsibilities of the Australia-wide Clinical Trials Jurisdictional Workshop are available on the Policies and Guidelines page.
Quality improvement/assurance and program evaluation
NSW Health encourages the widespread implementation of quality assurance (QA) programs as an essential part of a learning health care organisation. QA should be conducted as part of conventional clinical service delivery or ‘business-as -usual’. In almost all routine situations, QA projects should not require prior ethical review.
NSW Health adopts the distinctions between projects requiring ethical review and those that do not as set out in the NSW Health Guideline: GL2007_020 – Human Research Ethics Committees – Quality Improvement & Ethical Review: A Practice Guide for NSW.
• Researchers conducting human research are required to submit their projects to a Human Research Ethics Committee as per NSW Health Policy.
QA practitioners should assess the characteristics of their project against the NSW Health Guideline to determine if any of the ethical risks described within are present in their project statement.
- If so, review by an ethics review body should be sought. If advice is required, contact should be made with their local Clinical Governance Unit.
- If not, QA practitioners are free to commence their projects, after adhering to any institutional requirements. NSW Health policy is that ethics review and approval is not required merely for the mechanism of obtaining publication in a journal [see Q16 in GL 2007_020].
However, NSW Health observes that an increasing number of journals have required from QA practitioners a statement by an ethics review body prior to accepting a manuscript for publication.
To ensure that potentially valuable findings resulting from QA activities are communicated to the broader clinical governance community and that those findings are tested in the peer review literature; should a journal require a statement in such a circumstance, NSW Health has developed the attached letter which an ethics review body (including a Human Research Ethics Committee) may issue to QA practitioners retrospectively to state that, given the characteristics of the project statement, the project did not contain any of the ethical risks set out in the NSW Health Guideline, and that in accordance with NSW Health policy, was not required to be submitted for review by an ethics review body.
The letter itself is not a statement of ethical approval of the project.
-
Introductory Letter to HREC Chairs
DOCX - 70 KB
-
HREC Letter - QA Ethics Advice
DOCX - 26 KB
| Research | Quality Improvement/ Assurance | Program Evaluation | |
| Purpose | Develop or contribute to generalizable knowledge (e.g., testing hypotheses), test a hypothesis or establish clinical practice standards where none are accepted. | Improve the performance of a program, or service as judged by accepted standards or ensure it conforms with expected norms. | Assess the implementation and effectiveness of a specific program with the aim of monitoring a specific program or making policy adjustments. |
| Design | Develop or contribute to generalizable knowledge. | Not applicable to populations other than that under evaluation. | Provide feedback to program to improve that program. |
| Mandate | Activities not mandated by institution or program. | Activity mandated by institution or department/clinic as part of its operations. | Activity mandated by program as part of its operations. |
| Findings | Expected to answer a research question. Findings are not expected to directly affect institutional or programmatic practice. | Promptly improve a program/process/system. Findings are expected to directly affect institutional practice and may identify needed corrective actions. | Findings are expected to directly affect conduct of program and identify improvements. |
| Population | Usually involves a subset of individuals; generally, statistical justification for sample size is used to ensure endpoints are met. | Includes all or most receiving a particular treatment or process. | Information on all or most participants within or affected by receiving a particular treatment or undergoing a particular practice or process. |
| Benefits | Designed to contribute to generalizable knowledge, subjects may or may not directly benefit. | Designed to promptly benefit a process, program or system, Participants may directly or indirectly benefit from activities. | No direct treatment benefit to participants is expected. Evaluation aims to benefit the society and concentrates on program improvements. |
| Risks/ Burdens | May place subjects at risk and stated as such. | By design, do not increase patient’s risk, with exception of possible privacy/confidentiality concerns. | By design, do not increase patient’s risk, with exception of possible privacy/confidentiality concerns. |
| Dissemination | Dissemination of information usually occurs in research/scientific publications; results expected to develop or contribute to generalizable knowledge by filling a gap in scientific knowledge, developing hypotheses or supporting, refining or refuting results from other research studies. | Publication in peer-reviewed literature as original research is not necessarily an expectation. Dissemination of information does not necessarily occur beyond the institution evaluated; dissemination of information may occur in quality improvement publications; when published or presented to a wider audience, the intent is to suggest potentially effective models, strategies, assessment tools or provide benchmarks or base rates rather than to develop or contribute to generalizable knowledge. | Publication in peer-reviewed literature as original research is not necessarily an expectation. Dissemination of information to program stakeholders and participants may be posted to ensure transparency of results; when published or presented to a wider audience, intent is to suggest potentially effective models, strategies, assessment tools. |
| Testing/ Analysis | Usually designed to statistically prove or disprove a hypothesis. | Compare a program/process/system to an established set of standards; utilize descriptive statistics that demonstrate change/trends. | Quantitative and qualitative analysis may be used. |
| Timeline | Research may take considerable time. | Often has clear start and finish dates. | Often has clear start and finish dates. |
| External Funding | Mostly required. | Mostly not required. | Mostly not required. |
*This resource is based on national/international guidance compiled from:
Quality Assurance, Quality Improvement, or Program Evaluation by Michigan State University 21-11-2012
Quality Assessment and Quality Improvement FAQs by Stanford University AID-H161/4
Different of Research, Quality Improvement and Program Evaluation by the Department of Evaluation and Research Services 12-09-2011.
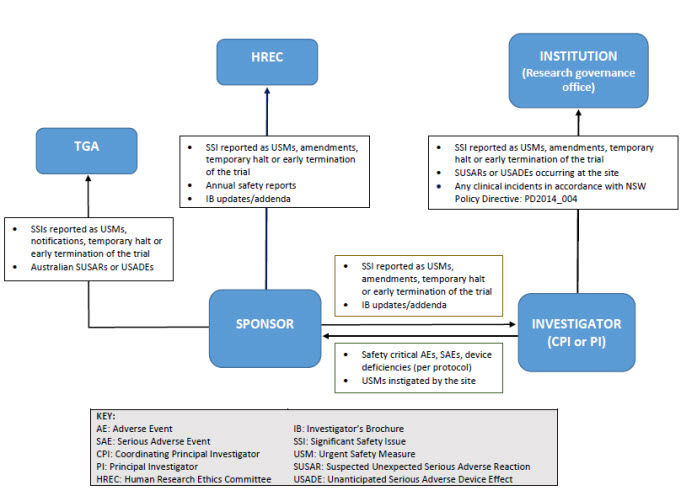
Safety notifications and reporting pathways for therapeutic goods trials
Safety notifications for therapeutic goods trials
A summary table of safety notifications to the Human Research Ethics Committee and Research Governance Officers
View safety notification summary table
- Type of event
- Who reports
- To whom
- When
- How
- Significant Safety Issue (SSI) implemented as an Urgent Safety Measure (USM)
- Sponsor/Delegate
- The reviewing HREC (and all investigators participating in the study)
- As soon as possible and no later than 72 hours of the sponsor becoming aware of the USM
- SSI Notification Form; Sponsor’s template
- Significant Safety Issue (SSI) not implemented as an Urgent Safety Measure (USM)
- Sponsor/Delegate
- The reviewing HREC (and all investigators participating in the study)
- Within 15 days of the sponsor becoming aware of the SSI
- SSI Notification Form; Sponsor’s template
- All Significant Safety Issues (SSIs)
- Principal Investigator
- The RGO for the site where the event occurred
- As soon as possible and no later than 72 hours of the PI becoming aware of the SSI
- SSI Notification Form; Sponsor’s template
- Suspected Unexpected Serious Adverse Events (SUSARs) and Unanticipated Serious Adverse Device Effects (USADEs) occurring at the site
- Principal Investigator
- The RGO for the site where the event occurred
- Within 72 hours of the PI becoming aware of the event
- SUSAR/USADE/URSAE Notification Form
- Investigator’s Brochure Updates/Addenda
- Sponsor/Delegate
- The reviewing HREC
- As and when updates are generated
- Submitted with a cover sheet or as part of an annual progress/annual safety report
- Annual Safety Report
- Coordinating Principal Investigator or Sponsor/Delegate
- The reviewing HREC
- Within annual progress report sent to the HREC or aligned with the safety reporting cycles of global companies
- Annual Progress Report or sponsor’s template
Reporting pathways for therapeutic goods trials
As illustrated below, sponsors may report directly to NSW Human Research Ethics Committee; however, they must ensure that all communications sent to the Human Research Ethics Committee adequately identify the trial and provide context in relation to the Human Research Ethics Committee’s role (e.g. whether there is any impact on patient safety, trial conduct or trial documentation).
Safety notifications and reporting pathways for non-therapeutic goods trials
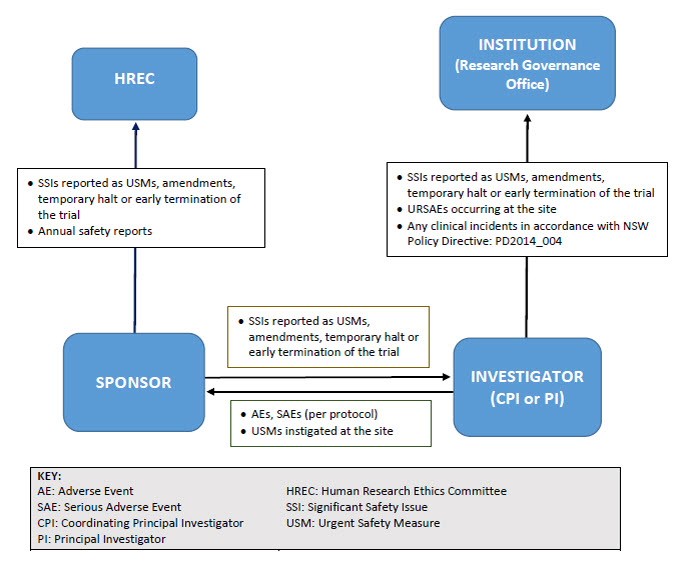
Safety notifications for non-therapeutic goods trials
View safety notification summary table
- Type of event
- Who reports
- To whom
- When
- How
- Significant Safety Issue (SSI) implemented as an Urgent Safety Measure (USM)
- Sponsor/Delegate
- The reviewing HREC (and all investigators participating in the study)
- As soon as possible and no later than 72 hours of the sponsor becoming aware of the USM
- SSI Notification Form; Sponsor’s template
- Significant Safety Issue (SSI) not implemented as an Urgent Safety Measure (USM)
- Sponsor/Delegate
- The reviewing HREC (and all investigators participating in the study)
- Within 15 days of the sponsor becoming aware of the SSI
- SSI Notification Form; Sponsor’s template
- All Significant Safety Issues (SSIs)
- Principal Investigator
- The RGO for the site where the event occurred
- As soon as possible and no later than 72 hours of the PI becoming aware of the SSI
- SSI Notification Form; Sponsor’s template
- Unexpected & Related Serious Adverse Event (URSAEs) occurring at the site
- Principal Investigator
- The RGO for the site where the event occurred
- Within 72 hours of the PI becoming aware of the event
- SUSAR/USADE/URSAE Notification Form
- Annual Safety Report
- Coordinating Principal Investigator or Sponsor/Delegate
- The reviewing HREC
- Annually (within the annual progress report)
- Annual Progress Report
Reporting pathway for non-therapeutic goods trials
As illustrated below, sponsors may report directly to NSW Human Research Ethics Committees; however, they must ensure that all communications sent to the Committee adequately identify the trial and provide context in relation to the Committee’s role (e.g. whether there is any impact on patient safety, trial conduct or trial documentation).
Resources and forms
Resources
-
Sample letter to Sponsor: Unwanted safety reports
DOCX - 28 KB
Forms
-
Significant Safety Issue Notification Form
DOCX - 51 KB
-
Local SUSAR/USADE/URSAE Notification Form
DOCX - 56 KB
Definitions
Therapeutic Goods Trials: Trials investigating the safety and/or effectiveness of medicines, biologicals or medical devices.
Non-Therapeutic Goods Trials: Trials other than a Therapeutic Goods Trial (e.g. radiotherapy, surgery, psychotherapy trials).
Suspected Unexpected Serious Adverse Reaction (SUSAR): An adverse reaction that is both serious and unexpected.
Unanticipated Serious Adverse Device Effects (USADEs): A serious adverse device effect which by its nature, incidence, severity or outcome has not been identified in the current version of the risk analysis report (and/or Investigator’s Brochure/Instructions for Use).
Urgent Safety Measure (USM): A measure required to be taken in order to eliminate an immediate hazard to a participant’s health or safety.
Significant Safety Issue (SSI): A safety issue that could adversely affect the safety of participants or materially impact on the continued ethical acceptability or conduct of the trial.
Unexpected & Related SAEs (URSAE): An adverse event that is:
- Serious – meets the definition of a serious adverse event
- Related – resulted from administration of the trial intervention
- Unexpected – the event is not described in the protocol as an expected occurrence.
FAQ Ethical and scientific review
Does student research require ethical review?
Yes. Student research involving humans that takes place in NSW public health organisations is subject to the same ethical and scientific review processes as other research.
Do pilot studies require ethical review?
Yes. Pilot studies involving humans that take place in NSW public health organisations are subject to the same ethical and scientific review processes as other research.
Do quality improvement projects require ethical review?
This depends on whether or not the quality improvement project poses any ethical risks to participants. The NSW Health Guideline GL2007_020 Human Research Ethics Committees – Quality Improvement & Ethical Review: A Practice Guide for NSW provides advice on determining when quality improvement activities require ethical review. The draft table “Distinction between Research, Quality Improvement/Quality Assurance and Program Evaluation” provides further guidance on these distinctions.
Individuals engaged in quality improvement projects should consult each NSW public health organisation involved in the project in order to identify the process adopted by that public health organisation for the ethical review of such projects.
What is the difference between full and expedited Human Research Ethics Committee review?
Human Research Ethics Committees established by NSW public health organisations provide full and expedited ethical and scientific review. Full Committee review must be undertaken for all human research that involves more than low risk to participants, as defined in the National Statement on Ethical Conduct in Human Research (2007). Expedited Committee review can be conducted for human research that only involves low or negligible risk to participants, as defined in the National Statement on Ethical Conduct in Human Research (2007).
Can I apply for expedited Human Research Ethics Committee review?
Only human research that involves low or negligible risk to participants, as defined in the National Statement on Ethical Conduct in Human Research (2007), is eligible for expedited Committee review. You should consult the NSW Health Human Research Ethics Committee associated with the site(s) at which you propose to conduct the research to determine if your project can be classified as low or negligible risk research before making an application.
Will a Human Research Ethics Committee automatically accept my application?
Human Research Ethics Committees can limit the number of research protocols they review at each meeting. If your application arrives after the limit has been met, the Committee will inform you that your application can be held over to the following meeting, or that you can apply to another Committee.
Investigators are encouraged to contact the Executive Officer of the Human Research Ethics Committee before submitting an application.
Some Human Research Ethics Committees will not accept an application if they are not certified to review research proposals in a particular area of research. For example, a Human Research Ethics Committee certified to review general research only, will not review applications for clinical trials. In addition, any Human Research Ethics Committee within NSW Health can advise an investigator that it does not have the appropriate expertise to review a particular project and suggest another, more appropriate Committee.
Also, when an investigator intends to submit an application as a low and/or negligible risk project for expedited Human Research Ethics Committee review, the Committee Executive Officer has the discretion to request that the research project be reviewed under full Committee review.
Can an application be withdrawn from one Human Research Ethics Committee and submitted to another?
An application can be withdrawn at any time prior to receipt of the Human Research Ethics Committee review outcome. Where an application is subsequently submitted to a different Committee, all documentation associated with the initial application and the reasons for withdrawal must accompany the submission. The new Human Research Ethics Committee can request that the application is re-submitted to the original Committee for any reason.
What is NSW Health's system of single ethical and scientific review of multi-centre research and how does it work?
Single ethical and scientific review of multi-centre research in NSW ensures that every research project that takes place in the NSW public health system is ethically and scientifically reviewed once only. NSW Human Research Ethics Committees certified through the National Health and Medical Research Council can carry out a single ethical and scientific review of multi-centre research on behalf of all sites within the NSW public health system.
Can investigators opt out of the system?
No. All multi-centre research projects that take place in the NSW public health system must be reviewed under the single ethical and scientific review system.
Does the system apply outside of NSW Health?
No. The NSW Health system of single ethical and scientific review is provided only for the benefit of NSW public health organisations. If the study is proposed to be conducted in more than one Australian public health organisation, the National Mutual Acceptance scheme applies. More information is available on the National Mutual Acceptance page.
Are there any exceptions to the single review principle?
Yes. The following types of research project have specific review requirements:
Research projects involving persons in custody or staff of NSW Justice Health & Forensic Mental Health Network
All human research projects involving persons in custody in NSW and/or staff of NSW Justice Health & Forensic Mental Health Network must be reviewed by the NSW Justice Health Human Research Ethics Committes.
Research projects specifically affecting the health and wellbeing of Aboriginal people and communities
Approval from the Aboriginal Health & Medical Research Council Ethics Committee is required where a research project involves research in, or concerning, NSW and any one of the following applies:
- the experience of Aboriginal people is an explicit focus of all or part of the research
- data collection is explicitly directed at Aboriginal people
- Aboriginal peoples, as a group, are to be examined in the results
- the information has an impact on one or more Aboriginal communities or
- Aboriginal health funds are a source of funding.
Clinical trials requiring access to statewide data collections
All research projects requiring access (including linkage) to statewide data collections owned or managed by NSW Health or the Cancer Institute NSW must be reviewed by the NSW Population & Health Services Research Ethics Committee.
The NSW Population and Health Services Research Ethics Committee is jointly convened by the Cancer Institute NSW and NSW Ministry of Health. The Committee is accredited in NSW as a lead Human Research Ethics Committee for population health and/or public health research. The Committee reviews:
- population health research utilising and/or linking routinely collected health (and other) data, including:
- data collections owned or managed by NSW Ministry of Health (e.g. NSW Admitted Patient Data Collection; Perinatal Data Collection; NSW Emergency Department Data Collection; and NSW Population Health Surveys)
- data collections owned or managed by the Cancer Institute NSW, (e.g. NSW Central Cancer Registry, NSW Pap Test Registry, BreastScreen Registry).
- applications from the NSW Ministry of Health and Cancer Institute NSW in relation to the conduct, management or modification of their data collections (referred to in 1 above).
Information including application forms and meeting dates can be found at NSW Population and Health Services Research Ethics Committee.
Where should I use the Human Research Ethics Application?
For applications to NSW and ACT public health Human Research Ethics Committees, the Human Research Ethics Application is located in both Online Forms and REGIS. As sites are at different stages in their adoption of REGIS, check with your local research office for guidance before submitting your ethics and site assessment applications.
Please do not create a Human Research Ethics Application for a NSW public health Human Research Ethics Committee through the National Health and Medical Research Council’s Human Research Ethics Application -generating website, because the ethics Committee will not be able to import and review the application.
What is the difference between REGIS and the National Health and Medical Research Council Human Research Ethics Applications?
Essentially the two forms are the same. Online Forms and REGIS use a licensed copy of the National Health and Medical Research Council’s Human Research Ethics Application. The questions are identical to those on the National Health and Medical Research Council’s Human Research Ethics Application.
How do I use the Human Research Ethics Application?
For applications in REGIS: The Question Reference Guide: Completing an Ethics Application gives step-by-step instructions for completing the Human Research Ethics Application in REGIS.
For applications in Online Forms: When you log in to Online Forms after 31 August 2017, select ‘Create New Project’ and then ‘HREA’. The Online Forms HREA is used in exactly the same way as the NEAF; complete the form, upload supporting documents, create SSAs, electronically authorise, and submit the application to the reviewing organisation. The Online Forms User Manual, which provides guidance on how to complete application forms and supporting documents required for making an application, will be updated to reflect the HREA. Under the Help menu, select User Manual.
What are the Human Research Ethics Application questions?
There is no published list of Human Research Ethics Application questions. The best way to get to know the Human Research Ethics Application is to log into Online Forms or REGIS, create a new Human Research Ethics Application, and to activate different questions to see what subsequent questions result. The National Health and Medical Research Council has provided question-specific guidance for many of the questions, which will be visible in the system.
I'm not sure how to answer some of the Human Research Ethics Application questions - who can I ask?
Your organisation’s research office can assist you with the content of your ethics application.
What if I accidentally created a Human Research Ethics Application at HREA.gov.au?
The Online Forms or REGIS websites must be used to complete and submit a Human Research Ethics Application to NSW Health Human Research Ethics Committees. If you have used another website to create a Human Research Ethics Application, contact the REGIS Helpdesk for assistance.
Importing the external Human Research Ethics Application into Online Forms might be possible in limited circumstances; you may contact the Office for Health and Medical Research’s Ethics and Governance Unit for more information.
What about applications for low and negligible risk studies?
The process for submitting an ethics application for LNR review has not changed. However, all applications will be submitted on a Human Research Ethics Application form regardless of risk level, because the Human Research Ethics Application has been designed as a smart form to accommodate all risk levels: you only need to answer questions which relate to the type of research you are proposing.
Human Research Ethics Application question 4.5 will ask under which review pathway you are intending to submit the application (greater than low risk, low risk, negligible risk). NSW Health Guidelines for Low and Negligible Risk (LNR) Research Review Processes or Exemption from Ethical Review provides further guidance on interpreting and clarifying some of the concepts contained in the National Statement.
When an investigator intends to submit an application as a LNR project for expedited Human Research Ethics Committee review, the Committee’s Executive Officer has the discretion to request that the research project be reviewed under full Human Research Ethics Committee review. This has not changed.
How will the Human Research Ethics Application affect the Site Specific Assessment?
The process for research governance/Site Specific Assessment remains the same under the Human Research Ethics Application.
What applications should I use to apply for Human Research Ethics Committee review under National Mutual Acceptance?
All National Mutual Acceptance Human Research Ethics Committees now use the Human Research Ethics Application. Please see National Mutual Acceptance for more information about applications submitted under that scheme (e.g. for multi-centre, multi-jurisdictional projects).
If you are submitting an ethics applications to a Human Research Ethics Committee that is located in a jurisdiction that is not currently part of National Mutual Acceptance (Northern Territory, Tasmania), please contact that Committee to obtain its local submission requirements.
What are other National Mutual Acceptance jurisdictions doing about low and negligible risk studies?
The Human Research Ethics Application must be used for any multi-jurisdiction research application where ethical approval is being sought under the National Mutual Acceptance system.
If I have a site in Victoria, do I still have to complete the Victorian Specific Module?
Yes. When the Human Research Ethics Application is used and the research project involves a site in Victoria, the Victorian Specific Module (VSM) must be uploaded as a supporting document to the Human Research Ethics Application and submitted to the reviewing ethics committee (even if the committee is outside Victoria).
Assistance and feedback on the HREA
Contact the National Health and Medical Research Council if you wish to provide feedback on the Human Research Ethics Application questions/question-specific guidance specifically.
Phone: 02 6217 9902
Email: help@hrea.gov.au
FAQ Clinical trial safety and monitoring
Will the new reporting requirements be implemented for trials with existing ethical approval?
Yes. The transition should be made as simple as possible and a Sample letter to Sponsors has been produced stating that the requirements apply to all new and existing trials (including trials opened before November 2016). The letter provides ‘blanket approval’ for the change so that individual protocol amendments to NSW Human Research Ethics Committees/Research Governance Officers will not be necessary.
The National Health and Medical Research Council’s Guidance only applies to therapeutic goods. How should other trials be handled?
NSW Health’s Policy Directive provides a framework for safety monitoring and reporting for non-therapeutic goods trials (e.g. surgery or psychotherapy) in order to standardise the requirements for all clinical trials. The same reporting principles and requirements that are described in the National Health and Medical Research Council’s Guidance have been applied to non-therapeutic goods trials.
Why has the Policy Directive used new terminology for non-therapeutic goods trials?
The terms ‘adverse reaction’ and ‘suspected unexpected serious adverse reaction’ (SUSAR) are not appropriate for device trials (as no reaction is taking place), so they are also not appropriate for non-therapeutic goods trials. The SUSAR-equivalent for a non-therapeutic good trial would be an Unexpected and Related Serious Adverse Event (URSAE).
What changes to reporting requirements have been made?
Human Research Ethics Committees will no longer receive single case AEs, SAE/SARs and SUSARs* or device/non-therapeutic good trial equivalents or six monthly line listings.
Human Research Ethics Committees will receive all significant safety issues, annual safety reports and investigator’s brochure updates.
Research Governance Offices will no longer receive single case AEs, SAE/SARs and external SUSARs* or device/non-therapeutic good equivalents or six monthly line listings.
Research Governance Offices will receive all significant safety issues (SSIs), any local SUSARs/USADEs/URSAEs and any research-related events that meet the definition of an incident (PD2014_004).
*Note: If a SAE/SAR/ SUSAR meets the definition of an SSI, it will be reported to the HREC/RGO through that reporting mechanism.
How will sponsors and investigators be informed of these changes?
NSW Health will confirm the changes to industry through Medicines Australia and the MTAA.
NSW Research Offices should communicate the changes as widely as possible to their own research active departments and any stakeholders they have contact with. NSW Research Offices may also direct stakeholders to NSW Health’s clinical trials webpage.
If the public health organisation is the sponsor, how should safety reporting be managed?
This is a business decision of the organisation. It is likely, as is current practice, that for clinical trials, many sponsor functions are delegated to the CPI or a coordinating centre. It is recommended that PHO-sponsors develop written procedures for their ‘sponsored’ trials so that all parties are aware of their responsibilities.
Sponsors should maintain oversight of functions that they delegate to ensure that they are being handled appropriately. This usually consists of some form of audit and monitoring activities to detect and rectify poor compliance.
Note: The NHMRC Guideline has not changed the role of the non-commercial trial sponsor. Instead, it has highlighted the requirements outlined in international Good Clinical Practice Guidelines adopted by the Therapeutic Goods Administration.
Will the use of standard safety reporting forms be mandated?
The use of two forms is mandated:
- Significant Safety Issue (SSI) Notification Form (reporting from sponsor to HREC)
- SUSAR/USADE/URSAE Notification Form (reporting from local investigator to their local RGO)
It is likely that these forms will evolve following validation (user acceptance testing). Please encourage users to provide real-time feedback on the utility of these forms (and any suggested improvements) so that relevant information can be provided for future versions. NSW Health will monitor the need to develop other standard forms but at present, sponsors may provide annual safety reports or updated IBs with a cover sheet.
Does every amendment relating to adverse events meet the definition of significant safety issue?
No, SSIs are safety issues that adversely affect the safety of participants or materially impact on the continued ethical acceptability or conduct of the trial. Normally, only issues that alter the risk benefit balance of the clinical trial meet the definition of a SSI.
An Urgent Safety Measure is defined as a measure required to be taken in order to eliminate an immediate hazard to the participant's health or safety. Do all urgent interventions that occur in a clinical trial meet the definition of an Urgent Safety Measure?
No. An urgent safety measure is an event that requires a change to trial procedures or the addition of unapproved trial procedures which are not defined in the protocol. Therefore, where urgent interventions are managed in accordance with the protocol, they are not considered USMs. For example an investigator may implement an immediate IMP dose reduction, in response to an observed toxicity, in accordance with the protocol’s dose modification rules.
How can HRECs monitor the welfare of the affected participant if they no longer get individual case reports such as SAEs and SUSARs?
In a single ethical review system as a trial progresses, the HREC’s responsibility is to keep under review the overall risk benefit balance of the trial, not the welfare of individual participants. The duty of care for individual participants lies with the Principal Investigator and their institution. Sponsors (and not HRECs) are responsible for monitoring that investigators are adequately executing their responsibilities in relation to participant safety.
Why was direct reporting (sponsor to Human Research Ethics Committee) introduced and should all NSW Human Research Ethics Committees accept direct reporting from the sponsor?
Yes. All NSW Human Research Ethics Committees and public health organisations should accept direct reporting in accordance with the National Health and Medical Research Council’s Guidance. The rationale for direct reporting is as follows:
Reduced administrative burden
Sponsors are finding it increasingly difficult to recruit CPIs due to the high administrative burden of the role. Much of that burden stems from the requirement for the lead site to transmit information generated by the sponsor to the Human Research Ethics Committee and also to other PIs.
By allowing the sponsor to send the information directly to the Human Research Ethics Committee, providing a copy in parallel to the CPI (and PIs where relevant), a two-step process becomes a single step process and the burden on the CPI is reduced.
In addition, allowing the sponsor to report directly reduces their monitoring burden as they no longer have to check (monitor) that the lead site has actually sent the report to the Human Research Ethics Committee.
Reporting timelines
The National Health and Medical Research Council’s Guidance requires sponsors to submit SSI reports within 72 hours (USMs) or 15 days (for other SSIs). Reporting via the CPI will make these timeframes harder to meet in some trials.
Alignment with national and international practice
Direct reporting is logical in countries operating a single ethical review model for multicentre trials (e.g. National Mutual Acceptance). Both the FDA and EU accept direct reporting and other states in Australia have already transitioned to direct reporting.
Note: Sponsors may delegate the task of ‘safety reporting to the HREC’ to the CPI or another third party. If sponsors choose to report directly, CPIs and PIs should be provided with any report/ information sent to the HREC that impacts on their role.
What advice should be provided to sponsors in relation to direct reporting to the Human Research Ethics Committee?
Sponsors should be made aware of two key requirements when reporting directly to NSW Human Research Ethics Committees:
- any direct reporting should take into account Section 5.2.20 of the National Statement. If sponsors limit their communication to the provision of reports, these communications will not influence (or be perceived to influence) the ethical review process
- sponsors must ensure that all reports/documents sent to the Human Research Ethics Committee (where standard templates are not available) adequately identify the trial and provide some context in relation to the Human Research Ethics Committee’s role; such as the impact on patient safety, trial conduct and/or trial documentation*
- sponsors must ensure that all reports/documents sent to the HREC (where standard templates are not available) adequately identify the trial and provide some context in relation to the HREC’s role; such as the impact on patient safety, trial conduct and/or trial documentation*.
*Note: Any changes to trial documents (e.g. protocols, PICFs, updated IBs) should be provided to the HREC using the standard amendment reporting process.
Can sponsors send HRECs an Investigator’s Brochure (IB) as an Annual Safety Report for therapeutic goods trials?
Yes. For therapeutic good trials, the National Health and Medical Research Council’s Guidance provides advice on the content of the annual safety report but allows flexibility; both in terms of the timing of that report and also the format.
For example, an annual safety report may consist of a cover sheet that cross-references the information contained within an IB/DSUR/DSMB report, which should be provided as an attachment. Annual safety reports must also:
- adequately identify the trial and provide some context in relation to the Human Research Ethics Committee’s role
- confirm that the safety monitoring plans described in the protocol/Human Research Ethics Committee application are being followed.
How should the Annual Safety Report for Non-Therapeutic Goods trials be provided to the HREC?
For non-therapeutic goods trials, the requirement for an annual safety report will be fulfilled by providing a summary of the trial’s evolving safety profile in the Annual Progress Report sent to the Human Research Ethics Committee.
What should HRECs do if they receive reports that are no longer required by the NHMRC Guidance?
Confirm receipt and inform the reporter that the event(s) sent are no longer required by NHMRC Guidance or NSW policy. These reports do not have to be reviewed by the committee.
Do investigators still need to print/review/file reports that are not required to be sent to sites by the National Health and Medical Research Council’s guidance?
No. The National Health and Medical Research Council’s guidance has aligned with EU Clinical Trial Regulation 536 which has removed the requirement for sponsors to send reports such as individual case SUSARs and six monthly line listings to investigators. However, sponsors may be obliged to follow global company policies that mandate the reporting of SUSARs/line listings. In these instances, it is good practice for investigator sites to confirm receipt of these reports and sites should follow sponsor guidance on how this may be achieved. The National Health and Medical Research Council’s guidance does not; however, oblige investigators to print, review and file ‘routine’ reports that have no bearing on participant safety or trial conduct.
Should the TGA receive all significant safety issues, even those that are solely due to trial conduct (and not the IMP/IMD)?
Yes. The TGA wishes to be notified of all events that are relevant to participant safety and therefore require notification of all SSIs, including those that have resulted from serious adverse events that are associated with a trial procedures. It should be noted however that the TGA should receive notification that a SSI has occurred but any resulting amendment (e.g. revising trial documentation) should be submitted to the HREC only.
What happens if, having read the Policy and these frequently asked questions, we have additional questions?
Please e-mail any queries to researchethics@doh.health.nsw.gov.au.
Updated 2 years ago