Device that rapidly images brains of stroke patients receives Medical Devices Fund grant
EMVision is developing rapid diagnostic imaging technology and is one of only three companies to receive funding in 2022 from the NSW Health Medical Devices Fund.
The sooner treatment begins after a stroke, the better the chances of survival and recovery. Yet effective treatment decisions rely on swift diagnostic imaging that reveals precisely what’s going on inside the patient’s brain. EMVision, a Sydney-based medical device company, is revolutionising the way strokes are diagnosed with the development of portable, high-speed neuro-imaging technology that could allow potentially life-saving decisions to be made faster. The technology has received $2.5 million from the NSW Health Medical Devices Fund (MDF) for a multi-site clinical trial which began in 2022.
Since 2013, the MDF has provided support to individuals, companies, public and private hospitals, medical research institutes, universities, and the medical devices industry, to take local innovation to market. To date, it has supported about 40 products with grants totalling more than $70 million.
EMVision co-founder and Executive Director Scott Kirkland says the support from the MDF will be instrumental to driving this key phase in the development of their technology.
“It’s a pivotal milestone for the company to be undertaking these trials and this funding goes a long way to support that,” he says.
Stroke is a leading cause of disability around the world. In Australia alone, more than 27,000 people will experience their first stroke each year, which equates to around one stroke every 19 minutes. During a stroke, a blood vessel or artery either bursts or is blocked, cutting off blood supply. Suddenly millions of brain cells start dying every minute, says Kirkland. “The earlier you can intervene, the quicker you can restore blood flow to minimise damage.”
Diagnostic imaging determines the location of the stroke and whether it’s being caused by a bleed or a blockage, which informs treatment decisions. In cases requiring highly specialised clot retrieval surgery, it can even determine which hospital to go to.
Imaging with CT or MRI is the current standard for stroke diagnosis, but their broader deployment is hampered by their large size. In most cases, the patient must be brought to the machine, says Kirkland. “When you have a time sensitive medical emergency like stroke, the best outcomes can be achieved when you bring the imaging to the patient to speed up decision making.”
Even the mobile versions of CTs can weigh up to 600 kilograms, he says, and while ultrasound technologies are much smaller, they aren’t suited for imaging brains because the ultrasound waves bounce off the skull.
“There’s a gap in portable brain imaging technologies,” he says. “Our technology in many ways is filling that gap …[it’s] portable, non-invasive, non-ionising, easy-to-operate, and gives quick valuable insights.”

Small but powerful
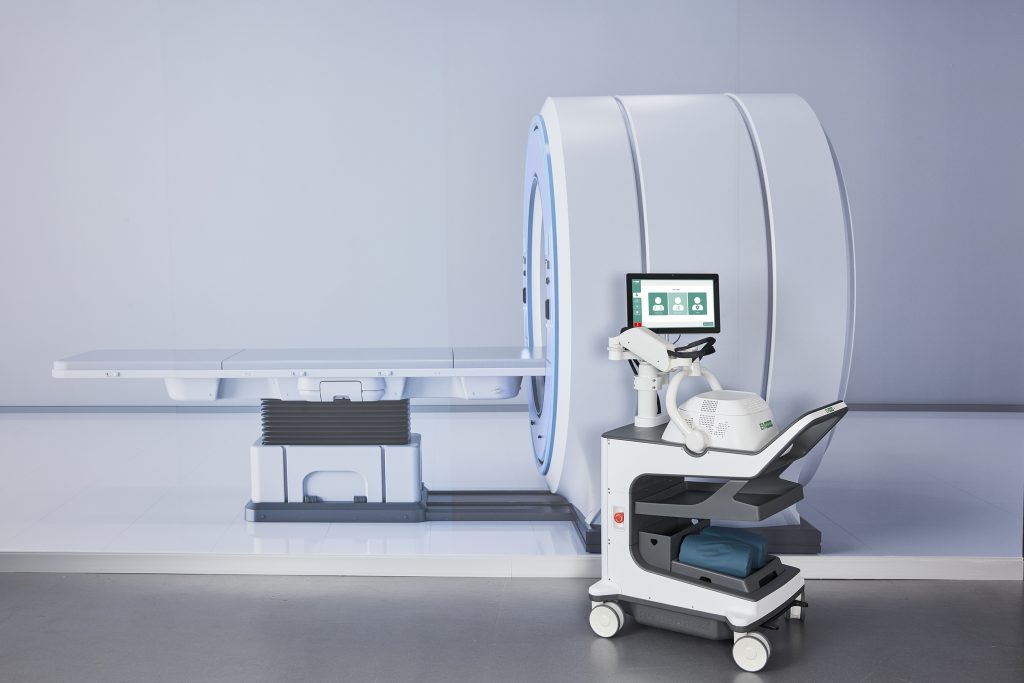
EMVision’s technology is a small-scale device that transmits low energy electromagnetic waves through a patient’s head. Because these waves are altered when they pass through abnormal brain tissue, the device can reveal distinct signatures of ischemic and haemorrhagic stroke. “The whole idea is it can operate at the bedside – you don’t need to move the patient,” says Kirkland. Moreover, the device’s high speed processing capability delivers results in just a few minutes.
There are two distinct generations of the technology. The 1st Generation is a portable, cart-based device that can be used in ICUs or a stroke ward, and could also be used in regional clinics with limited access to a CT. The device could also provide more frequent, less disruptive monitoring of patients in the ICU after treatment.
The 2nd Generation is an even smaller helmet device that could be used by paramedics in the field to facilitate treatment decisions on scene and better inform transfer decisions.
The $2.5 million awarded to EMVision from the NSW Health MDF will subsidise a multi-site clinical trial of the 1st generation cart-based device, enabling EMVision to test hundreds of stroke patients from the moment they arrive at the emergency room through to monitoring during treatment and recovery. The anticipated data generated over pre-validation and validation phases will be essential to gaining regulatory approval in Australia and abroad. “Clinical trial data is our currency,” says Kirkland. “It’s where the rubber hits the road.” The trial data will also support an expedited path to market for the helmet ambulance version.
Kirkland says the success of EMVision’s technology will deliver a wonderful return on investment by tackling a major global health economic burden, and creating more STEM jobs and manufacturing in NSW, where the company is based. Already they have experts and infrastructure set up for mechanical, electrical and software engineering, device assembly and quality control.
“The intention is to manufacture everything locally, right here in Macquarie Park.”
They’re all set up, and ready to grow.
Updated 2 years ago