clinicaltrialsNSW
enabling clinical trial capacity, capability and collaboration across New South Wales
clinicaltrialsNSW is an initiative of the Office for Health and Medical Research, NSW Health to enable clinical trial capacity, capability and collaboration across New South Wales (NSW).
We aim to:
- establish an entry point for clinical trials in NSW
- maintain policy directives for clinical trials within NSW Health, drawing on national and international best practice
- drive transformational change and improvements across the sector
- monitor and evaluate outcomes of clinical trial initiatives
Our team has national and international experience in:
- globally-sponsored multi-centre trials
- product innovation and commercialisation trials
- collaborative network trials
- investigator-initiated trials
- Phase I and First-in-Human trials
 |
 |
|
 |
 |
|
 |
 |
|
| Clinical Trials Register of Staff |
Useful resources
- Organisation
- Category
- Website
-
Australian Clinical Trials
- Government
- australianclinicaltrials.gov.au
-
A joint initiative between the National Health and Medical Research Council and the Department of Industry, Innovation and Science to provide information and resources to consumers, health care providers, researchers and industry about clinical trials.
-
SMEAssist
- Government
- tga.gov.au/sme-assist
-
A dedicated service that the Therapeutic Goods Administration (TGA) offers to help small to medium enterprises (SMEs), researchers, start-ups and those unfamiliar with regulation to understand their regulatory and legislative obligations.
-
Medicines Australia
- Industry peak body
- medicinesaustralia.com.au
-
Represents the discovery-driven pharmaceutical industry in Australia. Its member companies invent, manufacture and supply innovative medicines and vaccines to the Australian community.
-
Medical Technology Association of Australia
- Industry peak body
- mtaa.org.au
-
The national association representing companies in the medical technology industry. MTAA aims to ensure the benefits of modern, innovative and reliable medical technology are delivered effectively to provide better health outcomes to the Australian community.
-
AusBiotech
- Industry peak body
- ausbiotech.org
-
A well-connected network of over 3,000 members in the life sciences, including therapeutics, medical technology (devices and diagnostics), digital health, food technology and agricultural sectors. It has representation in each Australian state, providing a national network to support members and promote the commercialisation of Australian life science in national and international marketplaces.
-
ARCS
- Industry peak body
- arcs.com.au
-
A professional development association for people working in the development of therapeutic goods. It serves all personnel committed to therapeutics development in Australia. It also provides education and training to industry and non-industry personnel, with its educational events open to members and non-members.
-
ACTA
- Industry peak body
- clinicaltrialsalliance.org.au
-
A national peak body supporting and representing the networks of clinician researchers that conduct investigator-initiated or 'public-good' clinical trials within the Australian health system.
-
MTPConnect
- Industry peak body
- mtpconnect.org.au
-
A not-for-profit organisation aiming to accelerate the rate of growth of the medical technologies, biotechnologies and pharmaceuticals sector to increase commercialisation, collaboration and establish Australia as an Asia-Pacific hub for MTP companies.
-
CT:IQ
- Clinical trial initiative
- ctiq.com.au
-
A collaborative of stakeholders, all interested and involved in clinical trials in different ways, with a shared passion for striving for excellence in clinical trials.
-
TransCelerate
- Clinical trial initiative
- transceleratebiopharmainc.com
-
Create pragmatic solutions, such as templates, guidance, model frameworks and systems that addresses barriers and issues that are negatively impacting the ability of the biopharmaceutical industry to bring medicines to patients that need them. These solutions are developed collaboratively and can be voluntarily adopted by stakeholders in the clinical research ecosystem.
-
Clinical Trials Transformation Initiative
- Clinical trial initiative
- ctti-clinicaltrials.org
-
It is made up of more than 80 organisations from across the clinical trial enterprise. Members include representatives of government agencies, industry representatives, patient advocacy groups, professional societies, investigator groups, academic institutions, and other interested parties.
-
Therapeutic Goods Act 1989
- Therapeutic Goods Administration (TGA)
- legislation.gov.au/Series/C2004A03952
-
An Act relating to therapeutic goods in Australia.
-
Australian Register of Therapeutic Goods
- Therapeutic Goods Administration (TGA)
- tga.gov.au/artg
-
Therapeutic goods entered in the Australian Register of Therapeutic Goods (ARTG) can be lawfully supplied in Australia.
-
In-vitro diagnostic medical devices
- TGA medical device
- tga.gov.au/overview-regulatory-framework-ivds
-
In-vitro diagnostic medical devices (IVDs) are, in general, pathology tests and related instrumentation used to carry out testing on human samples, where the results are intended to assist in clinical diagnosis or in making decisions concerning clinical management. A regulatory framework commenced on 1 July 2010 that ensures all IVDs will undergo a level of regulatory scrutiny that is commensurate with the risks associated with their use.
-
Regulation of software as a medical device
- TGA medical device
- tga.gov.au/regulation-software-based-medical-devices
-
Medical devices are regulated in Australia by the TGA. This includes software and mobile ‘apps’ that meet the definition of a medical device.
-
Australian New Zealand Clinical Trials Registry
- Trial registries
- anzctr.org.au
-
An online registry of clinical trials being undertaken in Australia, New Zealand and elsewhere.
-
US NIH Clinical Trials Registry
- Trial registries
- clinicaltrials.gov
-
A database of privately and publicly funded clinical studies conducted around the world.
-
WHO Clinical Trial Registry
- Trial registries
- apps.who.int/trialsearch
-
The clinical trials search portal provides access to a central database containing the trial registration data sets provided by the registries listed on the right. It also provides links to the full original records.
-
NHMRC registered Human Research Ethics Committees
- Australian Human Research Ethics Committees
- nhmrc.gov.au/research-policy/ethics/human-research-ethics-committees
-
An investigator can submit to any public HREC in Australia.
The National Health and Medical Research Council (NHMRC) register has over 200 HRECs in organisations across Australia. Registration means that the HREC has notified the NHMRC of its existence and declared that it meets the requirements of the National Statement.
-
Bellberry Human Research Ethics Committee
- Australian Human Research Ethics Committees
- bellberry.com.au
-
Bellberry Limited is a national, private not-for-profit organisation providing streamlined scientific and ethical review of human research projects across Australia.
It hosts eight HRECs with extensive experience in providing ethics review and has rapid access to relevant scientific experts and specialists for adult early phase clinical trials.
-
Sydney Children’s Hospitals Network Human Research Ethics Committee
- Australian Human Research Ethics Committees
- schn.health.nsw.gov.au/research/ethics-governance/ethics
-
The Sydney Children’s Hospital Network Human Research Ethics Committee is responsible for ensuring the ethical and scientific acceptability of research conducted at sites within the network and other paediatric specific research referred to it by any public health organisation from NSW, ACT, VIC, QLD and SA.
It has extensive experience in providing ethics review and has rapid access to relevant scientific experts and specialists for paediatric early phase clinical trials.
-
Australian Taxation Office
- R&D tax incentive
- ato.gov.au/Business/Research-and-development-tax-incentive
-
The research and development (R&D) tax incentive encourages companies to engage in R&D benefiting Australia, by providing a tax offset for eligible R&D activities.
-
GrantConnect
- Funding
- grants.gov.au
-
A centralised publication of current and future Australian Government grant opportunities and grants awarded.
-
Health Consumers NSW
- Consumer engagement
- hcnsw.org.au
-
A not-for-profit organisation and registered health charity who represent the interests of patients, carers and their families in NSW
Standard Operating Procedures—frequently asked questions
What are Standard Operating Procedures?
Standard operating procedures (SOPs) are defined as:
detailed, written instructions to achieve uniformity of the performance of a specific function.
They are controlled documents (version numbered and dated) and are revision-sensitive with a formal release date and periodic revision.
Does GCP require sites to have SOPs?
Yes. ICH GCP (R2) E6 contains two key statements:
- Systems with procedures that ensure the quality of every aspect of the trial should be implemented (Principle 2.13) and;
- The investigator/institution… should implement procedures to ensure the integrity of the trial-related duties and functions performed and any data generated (4.2.6).
Do SOPs need to cover sponsor responsibilities?
The National Clinical Trials Governance Framework requires organisations sponsoring clinical trials to delineate sponsor and site activities (trial management versus trial conduct). SOPs for trial conduct are used for site activities.
Do SOPs need to work to ‘principles of GCP’?
Internationally, neither the FDA nor European regulatory authorities require trials to work to full GCP when they are not intended to support a marketing application.
SOPs should be written to facilitate all clinical trials by explicitly recognising that risk-proportionate procedures are appropriate for some trials. This does not mean that trial quality will be compromised as quality is often enhanced using Quality by Design principles that focus on activities that are ‘critical to quality’.
The SOPs should also be written to discourage over-interpretation of ICH GCP which can make trials more burdensome than they need to be.
What are controlled documents?
Controlled documents are essential for ensuring consistency and reliability in processes and operations. ISO9001:2015 Quality Management Systems states documented information shall be controlled to ensure it is available and suitable for use and is adequately protected to maintain accuracy and completeness.
This diagram explains the hierarchy of three types of controlled documents.
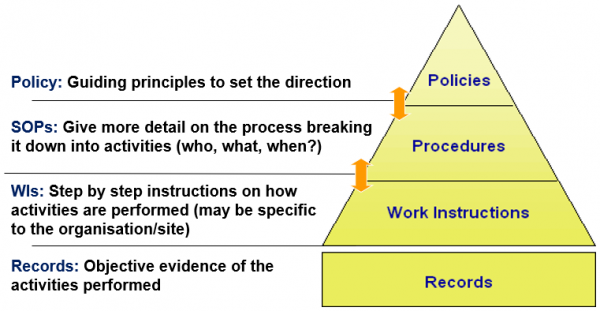
Diagram: hierarchy of three types of controlled documents
Early Phase Clinical Trials HREC Scheme—frequently asked questions
What is the definition of an early phase clinical trial (EPCT) in regard to the EPCT Human Research Ethics Committees (HRECs) Scheme?
Early phase clinical trials include all clinical trial phases up to but not including Phase II. It can also include studies with any Phase I component.
Read the quick reference guide below for more information.
What are the key changes of this scheme?
NSW Health is excluding all adult EPCT from the National Mutual Acceptance (NMA) model. All adult EPCT applications proposed to be conducted in a NSW public health organisation (PHO) site, must be submitted to Bellberry HRECs for ethics review.
Paediatric EPCTs with a NSW PHO lead site is required to be submitted to Sydney Children’s Health Network (SCHN) HREC for ethics review.
Read the quick reference guide below for more information.
Are paediatric EPCTs in NSW exempted from NMA model?
No, for multi-centred paediatric EPCTs, if an HREC hosted in a specialist paediatric tertiary hospital outside NSW has approved a paediatric EPCT, NSW PHO sites will continue to accept interstate HRECs’ approval in these instances.
Read the quick reference guide below for more information.
What is the age group within the scope of Bellberry HRECs for EPCTs?
- Trials involving adults equal to and greater than the age of 18 years; or
- Combined paediatric and adult trials involving young people and adults equal to and greater than 16 years.
What is the age group within the scope of SCHN HREC for EPCTs?
- Trials involving only children and young people under the age of 18; and
- Combined paediatric and adult trials involving children and young people under the age of 16 and young adults up to the age of 25.
Will Bellberry be acceptable as a lead HREC for NSW PHOs’ for non-early phase clinical trials?
No, the expression of interest (EOI) that Bellberry responded to and were successful, was specifically for reviewing EPCTs. There is currently no plan to expand Bellberry’s commission to non-EPCTs.
Is Bellberry recognised in the NMA scheme? Do/will other jurisdictions recognise Bellberry approvals for multi-centre trials with centres in their states? Or will multiple HREC approvals be required? For example, if I set up a Phase I trial in NSW and obtain HREC approval from Bellberry, can my colleague in Victoria use that approval or does they need to go to another NMA HREC?
All private (and many university) sites in NSW and other jurisdictions currently accept Bellberry approvals. This scheme expands the acceptance of Bellberry approvals to EPCTs in NSW public health organisations (PHOs) as well. We welcome PHOs in other jurisdictions to accept a Bellberry-approved EPCT, but this is up to the individual jurisdictions. This scheme does not establish Bellberry as an NMA HREC.
For combined Phase I/II trials where the Phase I component of the trial is complete, does the request for ethics review of the Phase II component need to be submitted to the NSW Health EPCT HRECs?
No. For combined Phase I/II trials where the Phase I component of the trial is complete, Phase II ethics review applications do not have to be submitted to NSW Health EPCT HRECs. They can be submitted through the normal state-based single ethical review system or NMA scheme.
-
Early Phase Clinical Trial HRECs Scheme Quick Reference Guide
PDF - 65 KB
Updated 8 months ago
For any enquiries, please reach out to the clinicaltrialsNSW team: