Adopting a range of non-animal testing approaches in drug development and medical research is a key priority for NSW Health
Moves to reduce animal testing in Australia and all over the world will change future approaches to clinical research. This global trend will advance the use of non-animal technologies, such as organoids (tissue cultures that replicate organ function). To stay ahead of the curve, NSW Health has created the Non-Animal Technologies Network (NAT-Net), recently announced by the Honourable David Harris MP, Minister for Medical Research.

Minister Harris outlined the NSW Government allocation of $4.5 million for a research program to reduce and replace animals in medical research using alternative models such as human-derived cells, tissue and recorded data for testing of medical procedures and drug therapies. Building on the existing NSW Government commitment to replacing and reducing the use of animals in medical research, NAT-Net will significantly enhance the state’s ability to accelerate scientific breakthroughs in animal-free testing models. These may exceed animal testing performance in some areas by more accurately modelling our human biology, providing insights more relevant to human health. They may also better support precision medicine by enabling some treatments to be more tailored to individual patients.
Creating a framework and infrastructure
The NAT-Net collaboration is supported by the Office for Health and Medical Research (OHMR) and is designed to bring together many sector stakeholders, key opinion leaders and experts to advise on required infrastructure and regulations as well as oversee pilot projects. Eight NSW institutions that are leaders in research of non-animal technologies form the foundational partners of NAT-Net. They include the Children’s Medical Research Institute, Hunter Medical Research Institute, University of New South Wales, University of Wollongong, University of Technology Sydney, University of Sydney, University of Newcastle and Victor Chang Cardiac Research Institute.
Taking the lead in non-animal technologies
In the future, the worldwide market and demand for non-animal testing models to be used in clinical trials will only continue to grow. This was confirmed at the recent breakfast showcase presented by NSW Health at the recent BIO 2024 convention in San Diego. “The standing room only event drew a very engaged audience keen to learn more about cutting edge non-animal testing models and the steps NSW Health is taking to embed them in clinical trials,” says Laura Collie, Senior Medical Advisor at OHMR, who presented at the breakfast event.

“A major driver of this change is occurring globally, for example in America and Europe,” says Collie. “As non-animal technologies have become more advanced, they are increasingly viewed as more valid options to replace and reduce animal testing. In parallel we also need to create new frameworks to oversee these novel testing approaches and ensure they are standardised and validated, which is why NAT-Net is so important.”
The three pillars of NAT-Net
The Non-Animal Technologies Network will oversee three major streams:
- The research pillar: This will involve four foundational research projects as well as a competitive grant program to accelerate development of innovative, effective and sustainable non-animal models in clinical research. “The NAT-Net research pillar will enable multi-organ models to be further investigated and developed,” says Collie. “At the moment many of these models replicate a single organ, but for some more complex diseases, it is beneficial to test the treatment on numerous organs. This process involves building multiple organs in one Petrie dish then testing them together, which could be very useful for investigating treatments for a condition such as cystic fibrosis. One example discussed at the BIO breakfast panel centred around making an eye and a brain that will link together and form an optic nerve.”
- The infrastructure pillar: “This will ensure development of new assets and leverage existing ones to advance capabilities in non-animal technology,” says Collie. This will include biobanking and tissue collection, the creation of a co-ordinated pipeline for non-animal testing models and improvement of data standards as well.
- The regulatory pillar: In collaboration with national regulatory agencies, the NAT-Net national framework will be created to govern increased use of human cell and computer models and support their use to reduce and replace animal testing.
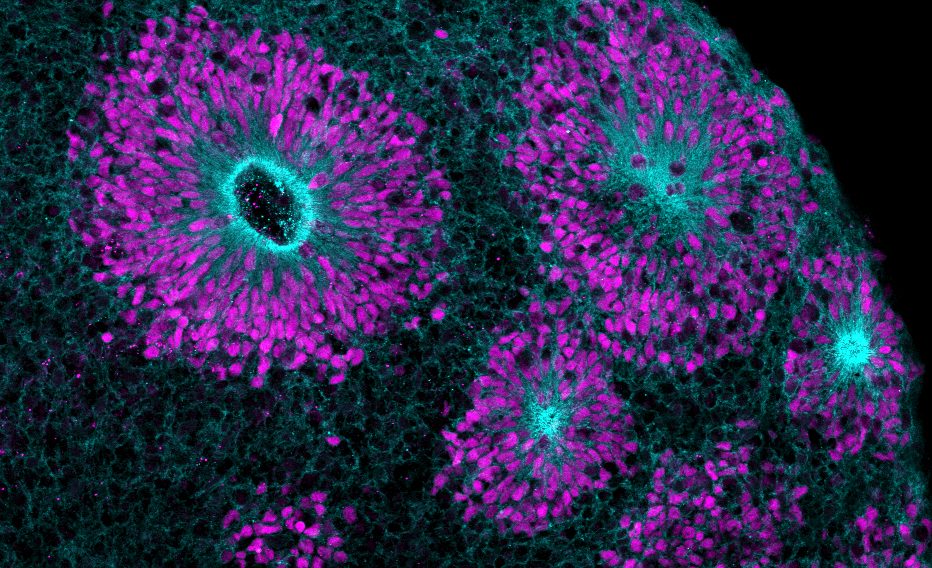
Human cell and tissue testing
Most non-animal technologies involve human tissues and cells. These have been donated with full patient consent via tissue banks, left-over biopsy samples and sometimes come from transplant organs that are unable to be utilised in a patient. In the lab these human tissues and cells are used to grow cell cultures. “Guidelines are in place to ensure they are safely and ethically collected, processed, handled and distributed for use in clinical trials,” Collie explains. Cell based non-animal technologies include:
– Organoids: These are 3D mini-organs grown from human stem cells. “Organoids are already being used in research to test treatments for diseases of the eye, brain, ear, kidney and heart,” Collie points out.
– Organs on a chip: These are miniature organoids with hollow channels made of flexible polymer that are lined with human stem cells and simulate human organ function. “For example, a lung-on-a-chip can carry out basic functions of the lungs and mimic airflow, bloodflow, inhalation and exhalation,” says Collie. “Organs on a chip have also been created to mimic the function of the stomach, liver, intestine, brain and skin.”
Computer modelling
This non-animal testing approach employs computers to simulate biological systems and predict their responses to potential and emerging therapies. Using mathematical models, DNA disease databases and health and scientific information, computers can conduct thousands of simulations. These then provide sophisticated answers to research questions about potential safety, hazards and side effects of new drugs, medical devices, surgical techniques and novel forms of treatment.
NSW Health supports a responsible humane approach to the use of animals for medical research, limiting use wherever possible with the highest standards of animal welfare. NAT-Net and the new non-animal testing applications will allow researchers to further minimise the ethical challenges of animal testing.
Updated 5 months ago