Australian start-up fighting an incurable side effect of cancer treatment
A start-up company, launched by two young Australian graduates from the University of Wollongong, is set to revolutionise the management of lymphoedema – a debilitating side effect of cancer treatment.
The company, Cenofex Innovations, is the brainchild of Drs Sheridan Gho and Michael Weaver. They are developing a wearable device that treats and manages the painful condition by massaging the lymphatic system, allowing patients to conduct normal lives.
There’s an urgent need for such a device. Lymphoedema is a life-long and incurable disease, and is most commonly caused by the removal of lymph nodes during cancer treatment, such as for breast, cervical, and ovarian cancer.
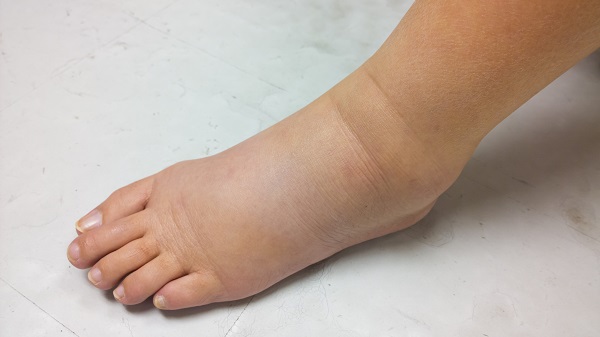
It results in the painful swelling of a limb or body region, and can affect- everything from a person’s immune system and mobility to their self-esteem and quality of life. There’s little known about what ultimately triggers it, and it can occur months or even years after cancer treatment.
As one patient told Cenofex, the effects can be profound: “When I heard I had breast cancer, I thought it was a death sentence. To have survived that, and then discover I have lymphoedema – I feel like I have been given a life sentence.”
A wearable solution
The new device in development by Cenofex uses ultrasound pulses to create a pressure differential that helps the lymphatic system move fluid around the body. It’s portable and wearable, which means patients can move around freely as it works.
Gho and Weaver are now pursuing commercialisation, and say the journey from the lab to the marketplace has been an exciting, if sometimes nerve-wracking, one.
“Yeah, it’s been quite a rollercoaster,” says Gho.
The key to their success, she says, is NSW Health’s start-up incubator, the Medical Device Commercialisation Training Program (MDCTP), designed to accelerate the commercialisation of medical technologies by building the knowledge, skills, and networks of the people behind the ideas.
When Gho first met Weaver, a mechatronics engineer skilled in medical radiation physics, they were part of the first cohort to go through the MDCTP in 2014.
“Being part of the MDCTP certainly opened our eyes and gave us the tools we needed to understand the journey of bringing a medical device to market,” says Gho, who studied biomechanics at the University of Wollongong, and started off designing sports bras for women recovering from breast cancer.
“Medical device companies would have to be one of the most challenging start-ups,” she says.
“There are just so many factors that you’ve got to take into consideration, outside of having a great product that’s addressing a really important need. That’s what the MDCTP really opened my eyes to.”
According to Weaver, the MDCTP did what his PhD work couldn’t – it got him through the significant learning curve that is commercialisation.
“While I was working at the university, I saw some amazing technologies and projects with great potential, but the mindset of academics is often not really prepared for the commercial world and getting things to industry,” he says.
A drive for change
For Gho and Weaver, the MDCTP was just the first step. After graduating from the program, they were selected to take part in the NSW Health funded NSW-QB3 Rosenman Scholar Program. As part of this Program they spent two years at QB3, the University of California’s hub for innovation and entrepreneurship in the life sciences in San Francisco, where they first launched Cenofex Innovations in 2016.
They returned to Australia in early 2018, to establish the company’s local arm in Sydney.
“Simply being in San Francisco with the ecosystem of start-ups there, was really great exposure for us,” says Gho. “It was a fantastic experience.”
“The whole environment lives and breathes start-up culture,” Weaver adds.
“You’d be at any café and you would overhear a conversation by these guys trying to do their own software start-up, or you’d have potential investors actually strike up a conversation with you. It’s just a completely different culture over there. You can understand why so many companies are born out of it.”
The next steps to get the Cenofex lymphoedema device on the market are getting through the clinical trials and stringent regulations that apply to new medical technology. But Gho says their plan is to launch it in Australia first.
“We really want to address the need here at home first, and essentially use Australia as a really good proving ground,” she says.
Australia is also considered one of the world leaders in lymphoedema research and the pair are now working with Macquarie University, which Weaver describes as “exceptionally good when it comes to lymphoedema.”
“So we’re pretty lucky to be able to have that opportunity to work with them,” he adds. “They’re going to help us to run the clinical trials through the testing methodologies and methods that they’ve already got established up there.”
While the time Gho and Weaver spent in San Francisco was enormously valuable, they say programs like the MDCTP can help affect real change in Australia.
“It’s a mindset,” says Gho. “I feel like here, to be a start-up company, it’s almost like the exception. Whereas in San Francisco, it wasn’t the exception, it was the norm.”
In 2018 they were awarded $1 million dollar from the Medical Devices Fund to continue the development and testing of the device. Gho and Weaver are now also exploring additional applications for their new device, such as for the treatment of injuries and other diseases associated with swelling.
Updated 4 years ago